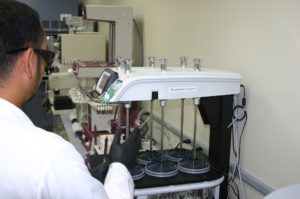
Excite Pharma Services offers dissolution testing for drug products and utilizes the USP Guidance (USP 711) for the design of its dissolution studies. Excite Pharma Services offers both the basket and paddle dissolution apparatuses as outlined in the USP guidance.
The purpose of dissolution testing is to provide critical in vitro drug release information for both quality control purposes (batch-to-batch consistency of solid oral dosage forms like tablets) and drug development purposes (to predict in vivo drug release profiles).
The rate at which the active ingredient is released is called the dissolution rate and is important to ensure the drug is delivered properly.
Dissolution testing is required for all dosage forms administered orally.
If you have any questions or are interested in more details of our dissolution capabilities, please contact us.
Helpful Links:
http://www.outsourcing-pharma.com/Community-Insights/The-role-of-dissolution-in-drug-development/
